Last accessed September 2019. ^2 ERLEADA product information Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210951s000lbl.pdf. Last accessed September 2019. ^3 European …
The FDA is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and …
All applications approved for the first time during the selected month. Includes New Molecular Entities (NMEs) and new biologics. Not all biologics are in Drugs@FDA. Does not include tentative approvals.
Select Devices@FDA to simultaneously search the PMN-510(k) Premarket Notification , PMA-Premarket Approval and HDE-Humanitarian Device Exemption.
Openness Score: Openness: 0 out of 5 Reason: URL extension "cfm" is an unknown format. Format field "HTML" receives score: 0. Last Updated: December 4, 2017
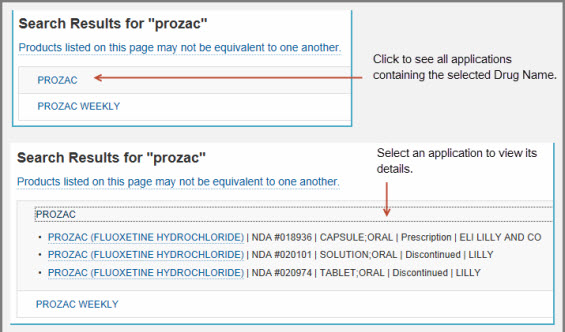
Drugs@FDA: FDA Approved Drug Products. Share · Tweet · Linkedin · Pin it; More … Download Drugs@FDA Express for free. Download iOS App Download …
No What Is It Oral-b Pro 1500 Rub It In Meaning rub it in definition: to make someone feel worse about something the person already feels embarrassed about: . Learn more. 11 Sep 2019 … rub it in definition: to make someone feel worse about something the person already feels embarrassed about: . Learn more. 100% Thc Our cannabis
Tobacco products listed with FDA. Note: Registration of an establishment, assignment of an FDA Establishment Identifier (FEI) number, or listing of a product does not constitute a jurisdictional determination, or an agency review or determination that the establishment or product is in compliance with FDA regulatory requirements.
Thc In Hemp Is cbd hemp oil LAS VEGAS, Sept. 12, 2019 /PRNewswire/ — Global Cannabinoids, the leading producers, manufacturers and distributors of American-Grown Hemp-Derived Cannabinoids in the U.S., today has signed a … The State of Colorado recently legalized the use of all parts of the hemp plant to be used in food products. A total of
Full prescribing information is available at: Nivolumab PI: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf Ipilimumab PI: …
On June 29, 2017, the U.S. Food and Drug Administration granted marketing approval to the praxis extended ras panel (Illumina … Praxis Extended RAS Panel is available at: …
Cbd Oil Review Forum The CBDOilUsers.com website and its companion CBD Oil Users Group on Facebook is dedicated to providing education, reviews, recommendations and the sharing of experiences among users of cbd products. rage reaction Image Aug 22, 2017 · How China’s most enduring meme has lasted a decade … you would’ve found it impossible to miss this unique style
 Modifications to the initial list of recognized standards, as published in the Federal Register, can be accessed at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/search.cfm. Please …
Modifications to the initial list of recognized standards, as published in the Federal Register, can be accessed at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/search.cfm. Please …
Approval Letter: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019a.pdf What is it? FoundationOne CDx (F1CDx) is a laboratory test designed to detect genetic mutations in 324 genes and two …
Matters described in fda warning letters may have been subject to subsequent interaction between FDA and the letter recipient that may have changed the regulatory status of issues discussed in the …
On December 1, 2017, the Food and Drug Administration approved Ogivri (trastuzumab-dkst, Mylan) as a biosimilar to Herceptin (trastuzumab … information about the approved uses: …
FDA regulates the sale of medical device products in the U.S. and monitors the safety of all regulated medical products. … The .gov means it’s official.